1. CHEMICAL CHARACTERIZATION
Identification and quantification of extractables and leachables (E&L)
Chemical characterization
Identification and quantification of extractables and leachables (E&L)
CHEMICAL CHARACTERIZATION FOR MORE SAFETY OF MEDICAL DEVICES
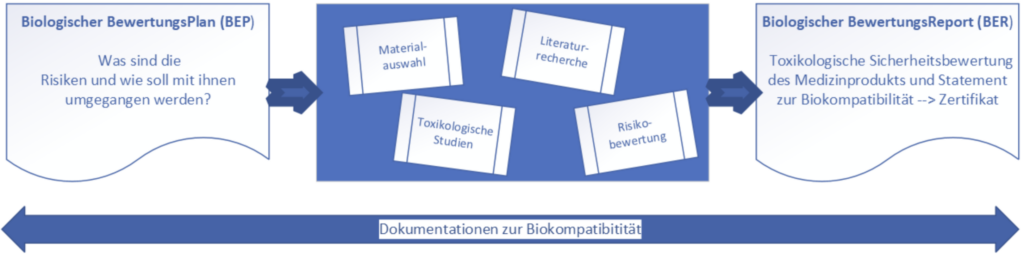
According to the biocompatibility standard ISO 10993-1, information on the chemical composition must be obtained for all medical devices. As a result, chemical characterization is required for most medical devices, from low-risk products such as plasters or medical spatulas to high-risk products such as implants. Analyzing the chemical substances released from a device into the patient’s body enables the prediction and reduction of potential toxicities.
WHICH PHYSICAL/CHEMICAL METHODS ARE SUITABLE FOR CHEMICAL CHARACTERIZATION?
In the chemical characterization of medical devices according to ISO 10993-18, volatile, semi-volatile and non-volatile organic and inorganic substances are analyzed using various methods and techniques. Using suitable methods these substances are extracted or washed out of the products and provide an overview of potentially undesirable substances released during use. This chemical profile is used to assess the overall risk to the patient or user when using the product.
The analytical methods used for this purpose are high-resolution and sensitive techniques. Depending on the hazard potential of the products, the following methods are usually used:
Inductively coupled plasma mass spectrometry (ICP-MS)
Analytical method used to screen for metallic and inorganic elements in the chemical characterization of medical devices.
Gas chromatography with vapor phase (head space) separation and coupled mass spectrometry (HS GC-MS)
Analytical method for the identification and quantification of volatile organic molecules (VOC).
Gas chromatography with coupled mass spectrometry (GC-MS)
Analytical method for the separation and identification of semi-volatile organic molecules (SVOC) up to a molecular weight of approx. 600 Da.
Liquid chromatography with coupled mass spectrometry (LC-MS)
Analytical method for the separation and identification of non-volatile organic molecules (NVOC) as part of the chemical characterization of medical devices.